Huge News – New Research, World-Class Advisors, and Global Partnerships
Huge News, Huge Progress
Thank you for your continued support of the NPHP1 Family Foundation. In this edition, we’re excited to share three big developments:
Work to test our gene therapy has officially begun with collaborators at Harvard’s Boston Children’s Hospital.
Our nine-member Scientific Advisory Board brings top-tier expertise from renowned academic institutions.
A trip to Paris to meet with partners advancing retinal disease treatments was a resounding success.
Your support makes all of this possible. Together, we are stopping a blinding disease in its tracks.
Getting Underway at BCH
We are working with Dr. Friedhelm Hildebrandt at Boston Children’s Hospital, a leading expert on NPHP genes and their associated conditions. Dr. Hildebrandt, along with Dr. Kraisoon Lomjansook and Dr. Ken Saida, is conducting natural history, dosing, and efficacy studies on two NPHP1 mouse models — one aggressive, one milder.
One of these mice has already been part of a successful proof-of-concept study in China. By replicating and expanding on that work, we will approach the FDA with robust data to support further testing and, eventually, a clinical trial.
Our Scientific Advisory Board
SABs are independent experts who advise and assess research collaborations, often volunteering their time. We’re proud to have assembled what we call “the New York Yankees of SABs”:
Johannes Birtel, MD, MBA — University of Oxford & University Medical Center Hamburg-Eppendorf; co-authored the largest study of NPHP1 retinal dystrophies.
Jefferson Doyle, MD, PhD — Johns Hopkins Medicine; pediatric ophthalmologist, genetic eye disease specialist, and Davidson’s doctor.
Cristhian Ildefonso, PhD — University of Florida; contributor to the Luxterna gene therapy program and retinal disease researcher.
Hemant Khanna, PhD — Astellas Gene Therapies; 20+ years in translational medicine and AAV-based gene therapy development.
Jens Konig, MD — University Children’s Hospital Münster; pediatric nephrologist, NEOCYST network leader, and renal ciliopathy researcher.
Jeffrey A. Moscow, MD — National Cancer Institute (retired); oncologist, drug developer, and longtime family friend.
Mark Pennesi, MD, PhD — Retina Foundation; director of ophthalmic genetics and clinical trial leader for inherited retinal diseases.
Jean Michel Rozet, PhD — Imagine Institute, Paris; leader in genetic decoding and therapy for severe retinal dystrophies.
Yang Sun, MD, PhD — Stanford Medicine; clinician-scientist using advanced technologies like CRISPR and optogenetics to treat blinding diseases.
Our Parisian Partners
While in Paris, we met with two promising organizations:
Medetia Pharmaceuticals — developing a small molecule treatment for retinal and renal diseases, based on discoveries by Dr. Sophie Saunier, who identified the NPHP1 gene. Davidson provided a blood sample for research.
Variant — developing a gene-modifier therapy for NPHP1, aiming for EU clinical trials in 2026.
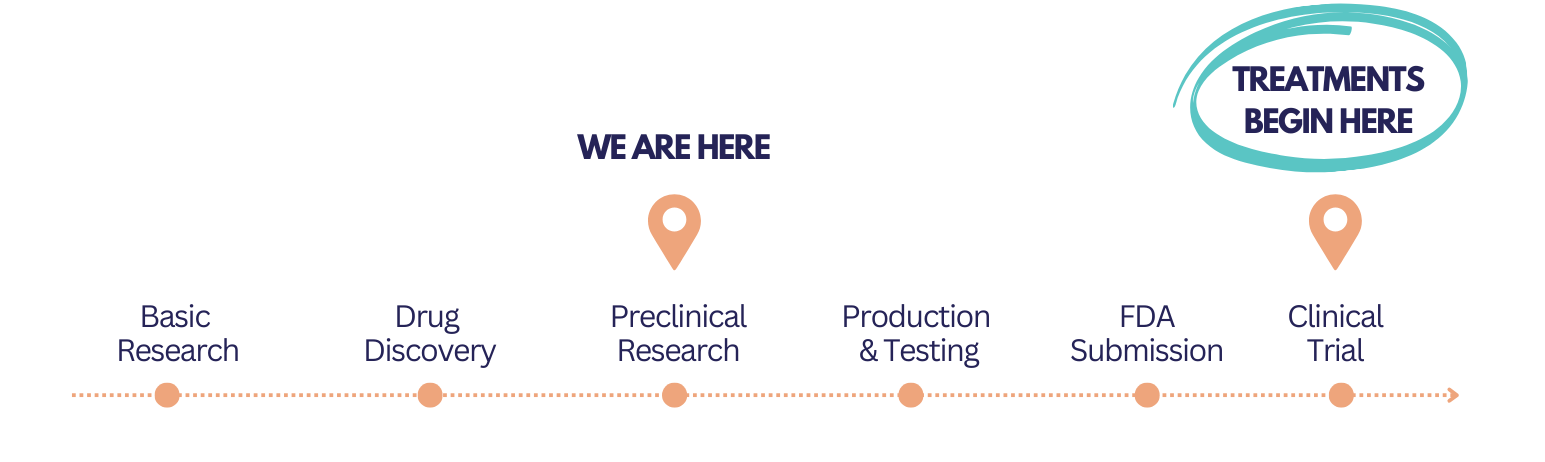
Up Next
By early fall, we expect to have mouse study data from Boston Children’s Hospital. From there, we’ll engage with the FDA while beginning the costly process of producing a human-grade version of our therapy.
We aim to celebrate a successful clinical trial by 2027 — and you’re invited to the victory party.